Completion & Production
New Chemistry Lifts Well Performance
By Martin “Marty” Shumway
HOUSTON–Oil and gas companies have restarted shut-in wells and are turning their attention back to resuming new well development activity. In the meantime, however, natural depletion continues to take a toll in fields everywhere, but especially in high-decline rate shale reservoirs. Like a shaken bottle of champagne that explodes when its cork is popped, shale wells come on fast with high initial production rates, but decline very rapidly within the first six months of production.
Unconventional wells are responsible for more than 60% of gross domestic oil and gas production, but without innovative technology, today’s level of production will be impossible to maintain. The Energy Information Administration reports that total U.S. oil output had slipped to 10.8 million barrels a day by the third week of August, down 2.3 million bbl/d from only five months earlier. To offset fast-depleting shale wells and replace falling production, producers have two choices: drill and complete new wells or extract more production out of existing wells. With either choice, they have to figure out how to do so in a lower oil price environment.
Operators have always turned to technology to make the most out of their existing producing wells. In today’s market, however, producers are under enormous pressure to operate within the limits of cashflow. More than ever, cashflow from producing wells is being counted on to fund new well investments and capitalize business operations, further reinforcing the need to not only optimize production, but cost effectively extract more production from every existing asset.
Biosurfactants are an exciting new area of technology development that help producers accomplish exactly that objective. These naturally produced biological solutions have unique reservoir-enhancing properties that boost production in existing wells and improve production in new well completions. All of this is delivered at lower dosage rates and lower cost than traditional surfactants. Laboratory and field data have demonstrated that biosurfactants can outperform traditional surfactants in mobilizing oil in both conventional and unconventional formations.
New Class Of Surfactant
Surfactants are the cornerstone of most of the chemical additives used in the oil field today–from drilling and completions fluids such as flowback aids, to production chemicals such as wax dispersants and reservoir stimulation formulations. Surfactants aid in recovering oil from the reservoir through wettability alteration and surface and interfacial tension reductions, effectively mobilizing oil by reducing the “drag” between oil and the surface of the reservoir rock.
Biosurfactants are amphiphilic molecules produced by microorganisms (yeasts, bacteria, and fungi) that use sustainable resources such as carbohydrates and natural oils as a carbon source. As 100% natural products, they are biodegradable and environmentally compatible. Equally important to oil well operators, they have many identified advantages over traditional surfactants, such as remarkably low critical micelle concentrations (very low effective dosage rates), very low toxicity, and high activities even at extreme temperatures, all at dosage rates of as little as one-fiftieth of traditional, synthetic chemical surfactants.
Moreover, biosurfactants can be used at pH values ranging from 2 to 12, and also tolerate high salt concentrations of 10% or more, whereas only 2% sodium chloride can inactivate traditional synthetic surfactants.
Biosurfactants have been effectively used for some time in a range of high-end applications, such as pharmaceutical drug delivery and household detergents, but they have not been commercially viable for the oil and gas industry. However, advancements in manufacturing technology now make them available in the volumes and at the pricing points needed for adoption at scale in oil and gas applications, with the focus on enhanced oil recovery, bioremediation and oil mobilization. The unique characteristics and multifunctionality of these green solutions offer producers an opportunity to extract maximum oil and gas from their assets at a fraction of the dosage rates and cost of synthetic surfactants.
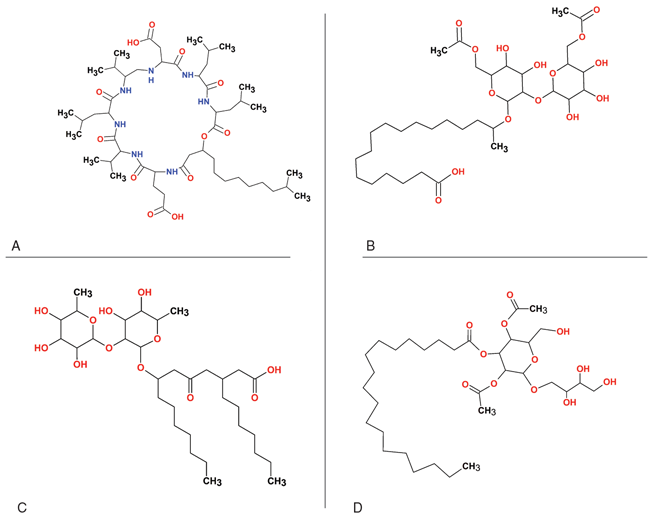
Figure 1 shows examples of the diverse chemical structures of key biosurfactants. Glycolipids are among the largest and most investigated groups of biosurfactants due to their higher fermentation yields and versatility in applications. Naturally produced surfactin molecules are shown in Panel A in Figure 1. Significant work also has been conducted on sophorolipids (Panel B), rhamnolipids (C), and mannosylerythritol lipids (D).
Benefits Of Biosurfactants
The benefits of biosurfactants include:
- Smaller micelle sizes to more effectively reach immobile oil;
- Faster and lower surface tensions to increase oil recovery;
- Adsorption/absorption for sustained production enhancement; and
- Remediation of wax deposits for increased production.
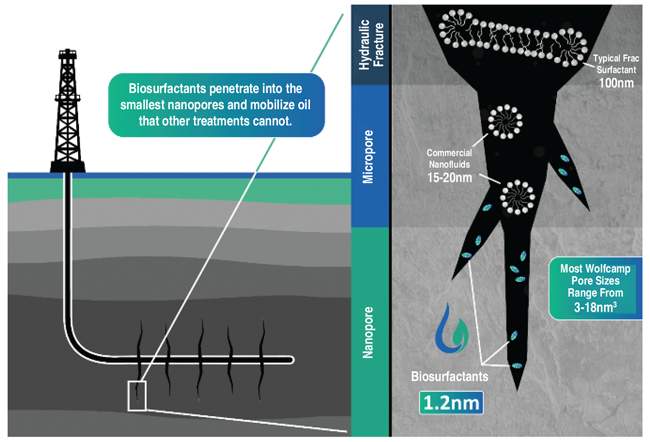
As illustrated in Figure 2, the smaller micelle size and lower dosage requirements allow biosurfactants to penetrate into the smallest nanopores and mobilize oil from even the tightest shale reservoirs. Some biosurfactants form micelles that are less than 2 nanometers in diameter, significantly smaller than the micelles formed by chemical surfactants and “nanoparticles.” This small micelle size is critical for performance when used to enhance recovery in unconventional tight formations where pore throats are very small. In the Wolfcamp Shale, for example, nanopores range from 3-18 nanometers.
Biosurfactants offer an unsurpassed ability to alter surface wettability, which is a key measure of their performance in oil recovery. Wetting refers to how a liquid deposited on a solid (or liquid) substrate spreads out, or the ability of liquids to form boundary surfaces with solid states. The degree of wetting is determined by measuring the contact angle, which the liquid forms in contact with the solids or liquids. The larger the wetting tendency, the smaller the contact angle, and consequently, the smaller surface tension is. A wetting liquid forms a contact angle smaller than 90 degrees with a solid, while a nonwetting liquid creates a contact angle between 90 and 180 degrees.
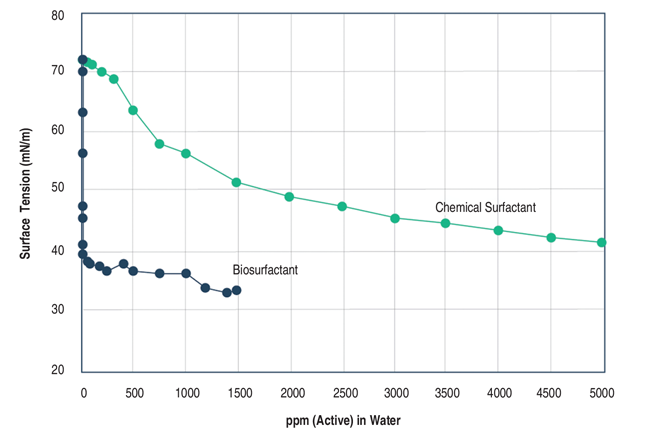
As noted, biosurfactants are amphiphilic compounds that are produced by living microbes, mostly on cell surfaces, or excreted extracellular hydrophobic and hydrophilic moieties that accumulate between fluid phases to reduce surface and interfacial tensions in a similar manner to chemical surfactants. The critical micelles concentration (CMC) of biosurfactants ranges from 1 to 2,000 milligrams per liter (mg/L), whereas interfacial (oil/water) and surface tensions are approximately 1 and 30 millinewton meters (mN/m), respectively. High-performing surfactants are able to reduce water surface tension from 72 to 35 mN/m and interfacial tension from 40 to 1 mN/m. Some biosurfactants can reduce the surface tension of water to 25 mN/m and interfacial tension to less than 1 mN/m.
In one study, the very low CMC of a biosurfactant was able to reduce surface tension of water at much lower concentrations when compared with a commercial chemical surfactant (a mixture of alcohol ethoxylates). Figure 3 presents the results. The study used the Du Noüy ring method of measuring equilibrium surface and interfacial tensions, one of three common methods based either on a force tensiometer ring or plate, or an optical pendant drop.
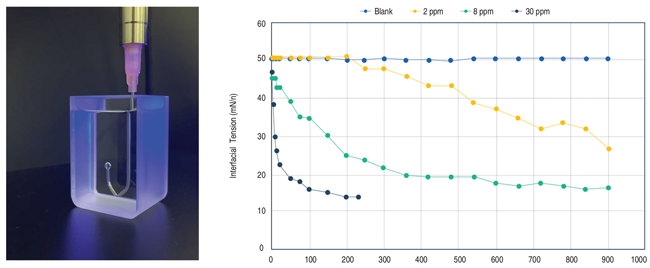
The efficacy of biosurfactants in reducing the interfacial tension of dodecane-water was demonstrated at various dosage rates as a function of time using the drop shape analysis method. The shape of the drop hanging from a needle is determined from the balance of forces, which include the surface tension of the liquid being investigated. As shown in Figure 4, the biosurfactant reduced interfacial tension from 50 to 13.5 mN/m at only 30 parts per million within 200 seconds.
Unlike synthetic surfactants, which do not absorb significantly onto reservoir rocks, up to 50% of biosurfactants are retained by the minerals in unconventional shale reservoirs. In addition to immediate production boosts, the biosurfactants slowly desorb over weeks and months to ensure long-term positive impacts on fluid properties and production rates after flowback.
Wax deposition remains a large focus and common issue, especially once waxy oils have been observed or hydraulic fracturing operations are performed. During hydraulic fracturing, often fluids at lower temperature than the reservoir are injected under pressure, potentially reducing oil temperature and causing wax precipitation. This situation can lead to a failure to reach the predicted maximum recovery, slow cleanup and decreased production.
Biosurfactants have been proven to exceed the wax dispersion efficacy of xylene and other hydrocarbon solvents, safely dispersing paraffin from rods and tubing and keeping it in suspension. This ultimately increases production rates by removing flow blockages. The subsequent slow release of biosurfactants inhibits paraffin redeposition for many months and mobilizes otherwise immobile oil to extending recovery benefits.
Innovative Solutions
Biosurfactants are currently formulated into products for paraffin dispersal, enhanced oil recovery and hydraulic fracturing fluids. Producers are using these products to increase returns on investments in new wells, improve oil recovery and extend the lifespan of producing assets. Certain biosurfactant treatments also qualify for state tax credits, giving operators a new avenue to cut costs. For example, one biosurfactant treatment program is approved as a tertiary EOR technology by the Texas Railroad Commission, which qualifies operators to receive a 50% severance tax reduction for 10 years on all oil (not just incremental oil) produced if a sustained production response is realized.
The challenging business conditions of 2020 are pushing the industry to adopt new technologies, and the future is reliant on innovative solutions that can meet the growing demands of higher oil production and greater recovery from existing reservoirs. Biosurfactants are providing timely answers to the needs of operators searching for cost-effective new solutions to the old problems of optimizing production and recovering more oil from the reservoir. These high-performing, robust and sustainable products are gaining recognition as the future of flow assurance and production technology while moving the industry forward in innovation, environmental stewardship, safety and cost.

Martin “Marty” Shumway is technical director for Locus Bio-Energy Solutions, which specializes in customized biosurfactant treatments for upstream oil applications. A licensed professional engineer and certified petroleum geologist, Shumway has more than 20 years of experience in the mining and petroleum industries. He holds a B.S. and an M.S. in engineering from The Ohio State University.
For other great articles about exploration, drilling, completions and production, subscribe to The American Oil & Gas Reporter and bookmark www.aogr.com.